Microchip-based capillary electrophoresis: sequencing and beyond

By Anthea Hammond, Ph.D.
From the advent of automated fluorescence DNA sequencing in 1985 to the draft sequence of the human genome published this year (1,2), DNA sequencing technology has come a long way. Development of capillary array electrophoresis (CAE) has provided an impressively high-speed, high-throughput sequencing method: the 14.8 billion base pairs of human genome so far sequenced were generated in just 9 months using the fully automated Applied Biosystems ABI Prism 3700 capillary sequencer, with 99.5% accuracy. CAE-based DNA sequencing is not without its limitations, however, and recent advances suggest that combining CAE with microchip technology may overcome many of these, with the additional advantage of still higher throughput.
Sequence analysis by capillary electrophoresis (CE) essentially involves the separation of fluorescently-labeled sequencing samples on hair-thin, 30-50cm long capillary gels. Recent advances in the type, composition and quality of the separating polymer, method of sample preparation, dye chemistry and automation have refined the process such that >1000 bases can be sequenced per hour in a single capillary.
Capillary windows (Source: Stanford University DNA Sequencing and Technology Center Website)
Operating an array of capillaries in parallel has increased throughput many fold, and both the ABI Prism 3700 and Molecular Dynamic's MegaBACE 1000 sequencer can analyze 96 sequences simultaneously. The major limitation of the CAE system is that its throughput is directly proportional to the number of separation capillaries in the instrument, and merely increasing this number leads to difficulties in controlling sample injection and detecting signals from all capillaries.
There are several compelling reasons to develop microchip devices for CAE-based sequencers, not least of which is their relatively low fabrication costs. Firstly, the intricate enclosed microchannels set in glass or fused silica substrates enable ultra-fast DNA separations due to unique sample loading formats, short separation distances and optimal thermal characteristics. Secondly, the photolithography techniques used to create these microchannels facilitate the addition of numerous capillaries, making ultra-high lane densities and throughput beyond that of capillary-array sequencers a possibility. Thirdly, precise optical positioning across an array of microchannels should be achievable. Lastly, integration of sample handling and analysis should reduce human interference and permit automation, further contributing to low running costs.
Microfabricated CE devices were established in 1992 (3), and the feasibility of using them for four-color DNA sequencing was first confirmed using single microchannels, with speeds of up to 580 bases in 18 minutes (4) and maximum read lengths of 800 bases (5). Significantly, Schmalzing et al (6) demonstrated that 'practical' DNA samples (rather than standard plasmid DNA) prepared for high-throughput, cost-sensitive sequencing under the Human Genome Project were analyzed with similar success; 500 bases in under 30 minutes. Importantly, this group found that no sample clean up was required, in contrast to conventional CE, which is highly sensitive to salt concentrations in the sample. Furthermore, long fragments were not prone to reduced signal strength, as is the case in CE systems, supporting the use of the microchip for high-throughput analyses.
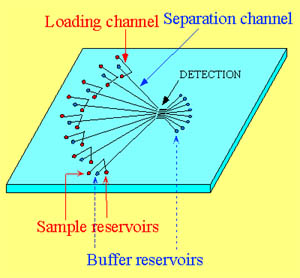
Multi-microchannel electrophoretic chip (Source: The Landers Group (James P. Landers) Department of Chemistry, University of Virginia, Charlottesville, VA)
In the last year, significant progress has been made towards DNA sequencing in parallel using multiple channel microchips, and towards automating this process. Backhouse et al (7), funded by PE Biosystems, have developed microchips with 48-188 independent 50cm-long microchannels. Using the 48-channel plate, they report resolution of over 600 bases with 98% accuracy, a performance similar to that of a conventional cylindrical capillary. Liu and colleagues (8), funded by Molecular Dynamics and Amersham Pharmacia Biotech, have developed a 16-channel CAE microchip with automated sample loading which sequences >500 bases to an accuracy of >99% in 18 minutes, including automated chip loading and sample injection. The robotic instrument automatically warms up the chip, loads samples from 96-well microtiter plates into the 16 channels using an 8-channel pipettor, moves the chip to the detection position, electrophoretically separates the samples, detects the separated fluorescent samples using a confocal detector, and exports the data for analysis.
Besides its promise for high-throughput DNA sequencing, microchip-based CE is also being exploited for other applications. Already commercially available are 'lab-on-a-chip' devices (Agilent/Caliper's 2001 Bioanalyzer and Caliper's Automated Fluidics System 90) for nucleic acid and protein analyses such as separation, sizing, quantifying and identifying DNA and RNA samples. Perhaps one of the most exciting applications of CE microchips is for the detection of sequence variations in specific genes. The vast resources provided by the Human Genome Project will rapidly improve our ability to identify genes responsible for human disease, necessitating low-cost, high-throughput methods to detect sequence variations in specific genes. Currently, DNA sequencing, allele-specific PCR (AS-PCR), single-stranded conformational polymorphism (SSCP) analysis, denaturing gradient gel electrophoresis, hybridization arrays and heteroduplex analysis (HDA) are the most widely used methods, and a major demand on all is the need for high-throughput versions. Recently, both SSCP (9) and HDA (10) have been developed in microchip CE systems and used to detect deletion, insertion and substitution mutations in the breast cancer susceptibility genes BRCA1 and BRCA2, with impressive efficiency. Furthermore, combining AS-PCR with HDA in a microchip CE device generated a powerful method for rapidly detecting specific mutations (11).
CE microchips have also been developed for rapid detection of single polynucleotide polymorphisms (SNPs) in the human genome. SNPs are the most common type of human genetic variation and are considered important tools for genetic testing, for identifying human disease genes, and for pharmacogenomics. Using an enzymatic mutation detection (EMDTM) method, analysis by microchip-based CE was highly accurate, required no sample cleanup and was 10 and 50 times faster than by conventional capillary and slab-gel electrophoresis (12).
In this post-genomics era, the refinement of multiple channel microchip-based CE devices and integrated process automation will undoubtedly establish this technology as a powerful high-throughput tool for invaluable genetic analyses.
References
- The Genome International Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature 409: 860-921 (2001)
- Venter JC et al. The Sequence of the Human Genome. Science 291:1304-1351 (2001)
- Manz A, Harrison DJ, Verpoorte EMJ, Fettinger JC, Paulus A, Ludi H and Widmer HM. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems: Capillary electrophoresis on a chip. J. Chromatography 593: 253-258 (1992)
- Salas-Solano O, Schmalzing D, Koutny L, Buonocore S, Adourian A, Matsudaira P, Ehrlich D. Optimization of high-performance DNA sequencing on short microfabricated electrophoretic devices. Anal. Chem. 72: 3129-3137 (2000)
- Koutny L, Schmalzing D, Salas-Solano O, El-Difrawy S, Adourian A, Buonocore S, Abbey K, McEwan P, Matsudaira P, Ehrlich D. Eight hundred-base sequencing in a microfabricated electrophoretic device. Anal. Chem. 72: 3388-3391 (2000)
- Schmalzing D, Tsao N, Koutny L, Chisholm D, Srivastava A, Adourian A, Linton L, McEwan P, Matsudaira P, Ehrlich D. Toward real-world sequencing by microdevice electrophoresis. Genome Res. 9: 853-858 (1999)
- Backhouse C, Caamano M, Oaks F, Nordman E, Carrillo A, Johnson B, Bay S. DNA sequencing in a monolithic microchannel device. Electrophoresis 21: 150-156 (2000)
- Liu S, Ren H, Gao Q, Roach DJ, Loder RT, Armstrong TM, Mao Q, Blaga I, Barker DL, Jovanovich SB. Automated parallel DNA sequencing on multiple channel microchips. PNAS 97: 5369-5374 (2000)
- Tian H, Jaquins-Gerstl A, Munro N, Trucco M, Brody LC, Landers JP. Single-strand conformation polymorphism analysis by capillary and microchip electrophoresis: a fast, simple method for detection of common mutations in BRCA1 and BRCA2. Genomics 63: 25-34 (2000)
- Tian H, Brody LC, Landers JP. Rapid detection of deletion, insertion, and substitution mutations via heteroduplex analysis using capillary- and microchip-based electrophoresis. Genome Res. 10: 1403-1413 (2000)
- Tian H, Brody LC, Fan S, Huang Z, Landers JP. Capillary and microchip electrophoresis for rapid detection of known mutations by combining allele-specific DNA amplification with heteroduplex analysis. Clin Chem. 47: 173-185 (2001)
- Schmalzing D, Belenky A, Novotny MA, Koutny L, Salas-Solano O, El-Difrawy S, Adourian A, Matsudaira P, Ehrlich D. Microchip electrophoresis: a method for high-speed SNP detection. Nucleic Acids Res. 28: E43 (2000)
About the author: Anthea Hammond, Ph.D., is a research scientist interested in virology and infectious disease. She currently works in the Molecular Medicine Program at the Mayo Clinic.
| Click here to subscribe to the free weekly Bioresearch Online newsletter. |
